Phase 1: Apheresis & Cell Separation
The initial phase of cell therapy production involves apheresis and cell separation. This phase is where peripheral blood stem cells or other cellular components are collected from the patient’s blood.

Phase 2: Transport
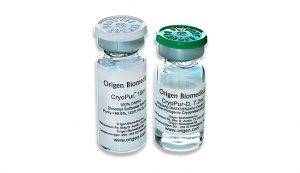
Transporting cellular components is a critical phase in cell therapy production, as maintaining cell viability and integrity during transit is essential for successful therapeutic outcomes. OriGen Biomedical offers specialized transport solutions like CryoStore Freezing Bags and CryoPur™ DMSO Solutions, designed to ensure cells are kept at optimal conditions throughout their journey. These products provide reliable cryopreservation, preserving cell integrity and minimizing the risk of damage.
OriGen Biomedical® CryoStore Freezing Bags and CryoPur DMSO Solutions create an environment that supports cell viability and functionality at their final destination. By leveraging these advanced solutions, laboratories can achieve consistent and reliable results, maintaining high-quality cellular material throughout the process. OriGen’s commitment to innovation and quality in transport solutions supports the future of regenerative medicine.

Phase 3: Gene Modification
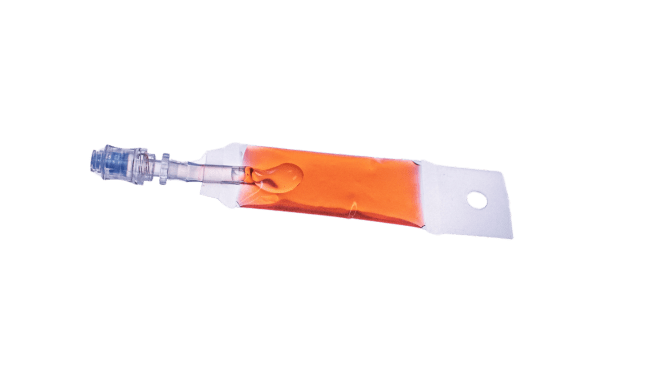
The gene modification phase is next for developing various cell therapies, including gene therapies. OriGen supports this phase with a range of products designed to ensure modified cells’ viability and therapeutic potential. OriGen Biomedical Accessory Sets Syringes are used for secure and aseptic fluid transfer, seamlessly integrating with closed-system processing. These syringes facilitate gene modification by offering reliable performance and ease of use, which is essential for researchers working on cutting-edge therapies.
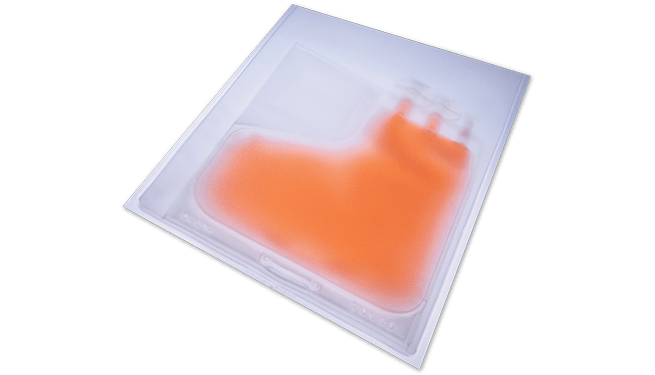
PermaLife® Cell Culture Bags provide an optimal environment for protecting and storing cells and tissues during gene modification. These bags maintain a stable environment for cell growth, enabling gas transmission and allowing easy microscopic observation without contamination risks. Researchers depend on OriGen Biomedical for consistent and reproducible results, confident in the performance and reliability of thoroughly tested and validated products.

Syringes for Use with OriGen Biomedical® Bags
PermaLife® Cell Culture Bags

Phase 4: Modified T-Cell Expansion
Expanding modified T-cells after gene modification is an important phase in producing many cell therapies, particularly in immunotherapy. OriGen Biomedical PermaLife Cell Culture Bags provide scalability and optimal growth conditions for CAR T-cells, enabling efficient scale-up of production. These bags facilitate the multiplication of genetically modified T cells, ensuring enough therapeutic T-cells are produced for patient infusion.
OriGen Biomedical PermaLife Mid-to-Large Cell Culture Bags and compatible syringes support the robust growth and proliferation of modified T-cells, maintaining cell health and functionality throughout the expansion process. By providing a controlled environment for cell growth, OriGen Biomedical products help achieve necessary cell counts while preserving therapeutic qualities, making them indispensable for advancing immunotherapy and other cell-based regenerative treatments.

Phase 5: Harvest Cells
The harvesting phase is where the expanded and modified cells are collected and prepared for final processing and application. OriGen Biomedical CryoStore Freezing Bags are used as the final product bag for cell therapy, ensuring safe transportation to the clinical site where the patient will receive treatment. For additional protection during transport and long-term storage, O-Wrap® Bags provide an extra layer of security, ensuring the primary cryogenic bags remain intact. Products like Manifold Sets, CryoPur Solutions, and Spike Adapters maintain the sterility and integrity of cell cultures, which are essential for producing safe and effective cell therapies.
OriGen Biomedical harvesting solutions are trusted by researchers and clinicians for their reliability and performance. These user-friendly products streamline the harvesting process, reducing the time and effort required. This efficiency helps laboratories maintain high throughput and consistent quality in their cell therapy production.

Manifold Sets for Closed System Processing
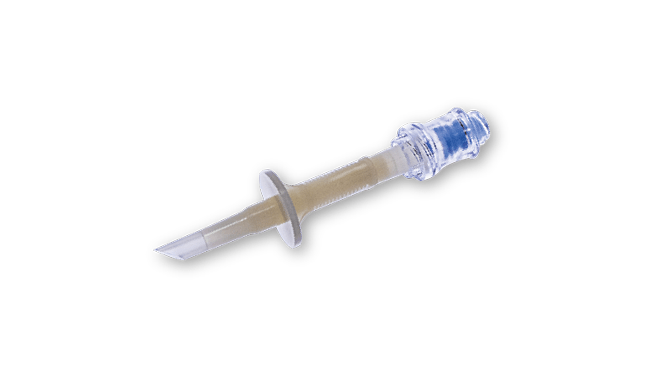
Spike Adapters for Closed System Processing

Phase 6: Transport

Phase 7: Infusion
Infusion is a critical step in cell therapy, where expanded CAR T-cells are administered to the patient through intravenous infusion. OriGen Biomedical products ensure these therapeutic cells retain their viability and functionality upon reaching the patient. CryoStore Freezing Bags protect CAR T-cells during storage and transport, while O-Wrap® Bags provide an extra layer of security, maintaining the sterility and integrity of the CAR T-cells.
During infusion preparation, OriGen Biomedical syringes and spike adapters enable precise and aseptic transfer of cells from storage bags to infusion devices. CryoPur™ Solutions support cell viability throughout cryopreservation, ensuring the CAR T-cells retain their therapeutic potential. By integrating seamlessly into existing workflows, these products help clinicians efficiently prepare and administer CAR T-cell therapy, maximizing treatment efficacy and increasing the likelihood of successful patient outcomes.